- Clinicians should be aware that a positive SARS-CoV-2 test result does not preclude influenza virus infection. For hospitalized patients with suspected influenza who are started on empiric antiviral treatment with oseltamivir, use of influenza molecular assays (Table 3) or multiplex assays that detect both influenza viruses and SARS-CoV-2 (Table 4) can inform clinical management.
- Clinicians should be aware that a positive influenza test result does not preclude SARS-CoV-2 infection. For hospitalized patients with a positive influenza test result, antiviral treatment of influenza with oseltamivir should be started as soon as possible, and clinicians should also follow guidelines for diagnosis and treatment of community-acquired pneumonia (community acquired pneumonia treatment guidance for adults: Metlay, 2019 ) and other respiratory infections, including SARS-CoV-2 infection (NIH COVID-19 treatment guidelines and IDSA COVID-19 treatment guidelines ) if clinically indicated, while awaiting SARS-CoV-2 testing results. Oseltamivir does not have in-vitro activity against SARS-CoV-2 (Choy, 2020 ).
Antiviral medications with activity against influenza viruses are an important adjunct to influenza vaccine in the control of influenza.
- Influenza antiviral prescription drugs can be used to treat influenza, and some can be used to prevent influenza.
- Six licensed prescription influenza antiviral drugs are approved in the United States.
- Four influenza antiviral medications approved by the U.S. Food and Drug Administration (FDA) are recommended for use in the United States.
- Three drugs are chemically related antiviral medications known as neuraminidase inhibitors that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses: oral oseltamivirphosphate (available as a generic version or under the trade name Tamiflu®), inhaled zanamivir (trade name Relenza®), and intravenous peramivir (trade name Rapivab®).
- The fourth drug is oral baloxavir marboxil (trade name Xofluza®), which is active against both influenza A and B viruses but has a different mechanism of action than neuraminidase inhibitors. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication.
- More information regarding the four recommended antiviral medications is available: Table 1.
- In hospitalized adults with influenza, early treatment with oseltamivir has been reported to reduce in-hospital death and the duration of hospitalization in some observational studies.
- In hospitalized children, early antiviral treatment with oseltamivir has been reported to shorten the duration of hospitalization in some observational studies.
- Clinical benefit is greatest when antiviral treatment is administered early, especially within 48 hours of influenza illness onset.
Table 1. Antiviral Medications Recommended for Treatment and Chemoprophylaxis of Influenza
Antiviral Agent Activity Against Use Recommended For Not Recommended for Use in Adverse Events Oral
OseltamivirInfluenza A and B Treatment Any age 1 N/A Adverse events: nausea, vomiting, headache. Post marketing reports of serious skin reactions and sporadic, transient neuropsychiatric events 2 Chemo- prophylaxis 3 months and older 1 N/A Inhaled
ZanamivirInfluenza A and B Treatment 7 yrs and older 3 People with underlying respiratory disease (e.g., asthma, COPD) 3 Adverse events: risk of bronchospasm, especially in the setting of underlying airways disease; sinusitis, and dizziness. Post marketing reports of serious skin reactions and sporadic, transient neuropsychiatric events 2 Chemo- prophylaxis 5 yrs and older 3 People with underlying respiratory disease (e.g., asthma, COPD) 3 Intravenous
PeramivirInfluenza A and B 4 Treatment 6 months and older 4 N/A Adverse events: diarrhea. Post marketing reports of serious skin reactions and sporadic, transient neuropsychiatric events 2 Chemo- prophylaxis 5 Not recommended N/A Oral
BaloxavirInfluenza A and B 6 Treatment 5 yrs and older 6 N/A Adverse events: none more common than placebo in clinical trials Chemo- prophylaxis 6 Approved for post-exposure prophylaxis in persons 5 yrs and older 6 Abbreviations: N/A = not applicable, COPD = chronic obstructive pulmonary disease.
- Oral oseltamivir phosphate is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 14 days and older, and for chemoprophylaxis in people 1 year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by the CDC and the American Academy of Pediatrics. If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical due to limited data in this age group.
- Self-injury or delirium; mainly reported among Japanese pediatric patients.
- Inhaled zanamivir is contraindicated in patients with underlying airways disease such as asthma or chronic obstructive pulmonary disease, and those with a history of allergy to lactose or milk protein.
- Intravenous peramivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 6 months and older. Peramivir efficacy is based on clinical trials versus placebo in which the predominant influenza virus type was influenza A; in one trial, a very limited number of subjects infected with influenza B virus were enrolled.
- There are no data available for use of peramivir for chemoprophylaxis of influenza.
- Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people aged ≥5 years who are otherwise healthy, or in people aged ≥12 years who are high risk of developing influenza-related complications. Baloxavir efficacy for initial FDA approval in October 2018 was based on clinical trials in previously healthy outpatients 12 to 64 years old (Hayden, 2018 ). Singledose baloxavir t reatment was superior to placebo and had similar clinical efficacy in time to alleviation of symptoms to a 5-day treatment course of oseltamivir.
In October 2019, FDA approved an indication for baloxavir treatment of acute uncomplicated influenza within 2 days of illness onset in people 12 years and older at high risk of developing influenza-related complications, based upon the findings of a clinical trial (Ison, 2020). In this clinical trial of early initiation of antiviral treatment for uncomplicated influenza in highrisk patients, baloxavir was superior to placebo and had similar overall efficacy to oseltamivir in the time to alleviation of symptoms. For patients with influenza B virus infection, baloxavir significantly reduced the median time to improvement of symptoms compared with oseltamivir by more than 24 hours.
For patients with influenza B virus infection, baloxavir significantly reduced the median time to improvement of symptoms compared with oseltamivir by more than 24 hours. However, there are no available data for baloxavir treatment of influenza in pregnant people, immunocompromised people, or in people with severe influenza who are not hospitalized.
A randomized clinical trial reported that combination neuraminidase inhibitor (primarily oseltamivir) and baloxavir for treatment of hospitalized influenza patients aged ≥12 years did not result in superior clinical benefit (time to clinical improvement) compared with neuraminidase inhibitor and placebo (Kumar, 2022 ).
In November 2020, FDA expanded approval of baloxavir to include post exposure prophylaxis of influenza for persons aged ≥12 years within 48 hours of contact with an individual with influenza, based on the findings of a clinical trial among household contacts of index patient with influenza (Ikematsu, 2020 ). In this study, baloxavir post-exposure prophylaxis (PEP) of influenza in household members (19% were younger than 12 years; 73% received baloxavir within 24 hours of onset of symptoms in the index household case who received antiviral treatment) significantly reduced the risk of laboratory confirmed by 86% among those who received baloxavir PEP than among those who received placebo (1.9% [7 of 374] vs. 13.6% [51 of 375]; adjusted risk ratio, 0.14; 95% confidence interval [CI], 0.06 to 0.30; P<0.001).
In August 2022, FDA expanded approval of baloxavir for post-exposure prophylaxis of influenza in persons aged 5 years and older within 48 hours of contact with an individual with influenza package insert XOFLUZA [963 KB, 22 pages] .
Summary of Influenza Antiviral Treatment Recommendations- Antiviral treatment is recommended as early as possible for any patient with confirmed or suspected influenza who:
- is hospitalized;*
- has severe, complicated, or progressive illness;* or
- is at higher risk for influenza complications.
*Oral or enterically administered oseltamivir is the recommended antiviral for patients with severe, complicated, or progressive illness who are not hospitalized, and for hospitalized influenza patients. For hospitalized patients who cannot tolerate or absorb oral or enterically administered oseltamivir because of suspected or known gastric stasis, malabsorption, or gastrointestinal bleeding, intravenous peramivir may be considered (Lee, 2017; de Jong, 2014; Ison, 2014; Ison, 2013). There are insufficient data to support general use of inhaled zanamivir and intravenous peramivir in patients with severe influenza disease. There are no available data from clinical trials on use of baloxavir treatment in patients with severe influenza disease who are not hospitalized.
**Oral oseltamivir and oral baloxavir are available treatment options for patients at higher risk for influenza complication depending upon their underlying conditions and age (Table 1). Data on use of peramivir or zanamivir are very limited in high-risk outpatients with influenza.
- Oral oseltamivir is preferred for treatment of pregnant people (Rasmussen , 2011 ). Pregnant people are recommended to receive the same antiviral dosing as non-pregnant people. Multiple studies have reported safe use of neuraminidase inhibitors during pregnancy (Dunstan, 2014 ; Xie, 2013 ; Saito, 2013 ; Wollenhaupt, 2014 ; Beau, 2014 ; Svensson, 2011 ; Greer, 2010 ; Graner, 2017 ); Ehrenstien, 2018 ; Chambers, 2019 ; Bennekom, 2019 ; ACOG Committee, 2018 ). See Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for additional information. Baloxavir is not recommended for the treatment of influenza in pregnant or breastfeeding people, as there are no available efficacy or safety data for baloxavir in this pregnant people (Chow, 2021 ), and no available data on the presence of baloxavir in human milk, the effects on the breastfed infant, or the effects on milk production.
- CDC does not recommend use of baloxavir for monotherapy of influenza in severely immunosuppressed persons. There are no available efficacy, safety, or resistance data for baloxavir monotherapy of influenza in severely immunosuppressed patients and emergence of resistance during treatment is a concern because of prolonged influenza viral replication in these patients.
- When indicated, antiviral treatment should be started as soon as possible after illness onset, ideally within 48 hours of symptom onset for the greatest clinical benefit. However, observational studies have reported that antiviral treatment of influenza can have clinical benefit in patients with severe, complicated or progressive illness, and in hospitalized patients when started after 48 hours of illness onset.
- Decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza (see resources regarding Clinical Description and Lab Diagnosis of Influenza for more information on influenza diagnostic testing).
- Clinical benefit is greatest when antiviral treatment is started as close to illness onset as possible.
- The recommended treatment course for uncomplicated influenza is two doses per day of oral oseltamivir or inhaled zanamivir for 5 days, or one dose of intravenous peramivir or oral baloxavir for 1 day.
- While influenza vaccination is the best way to prevent influenza illness, a history of influenza vaccination does not rule out the possibility of influenza virus infection in an ill patient with clinical signs and symptoms compatible with influenza.
Figure: Guide for considering influenza testing and treatment when influenza viruses are circulating in the community (regardless of influenza vaccination history) 1
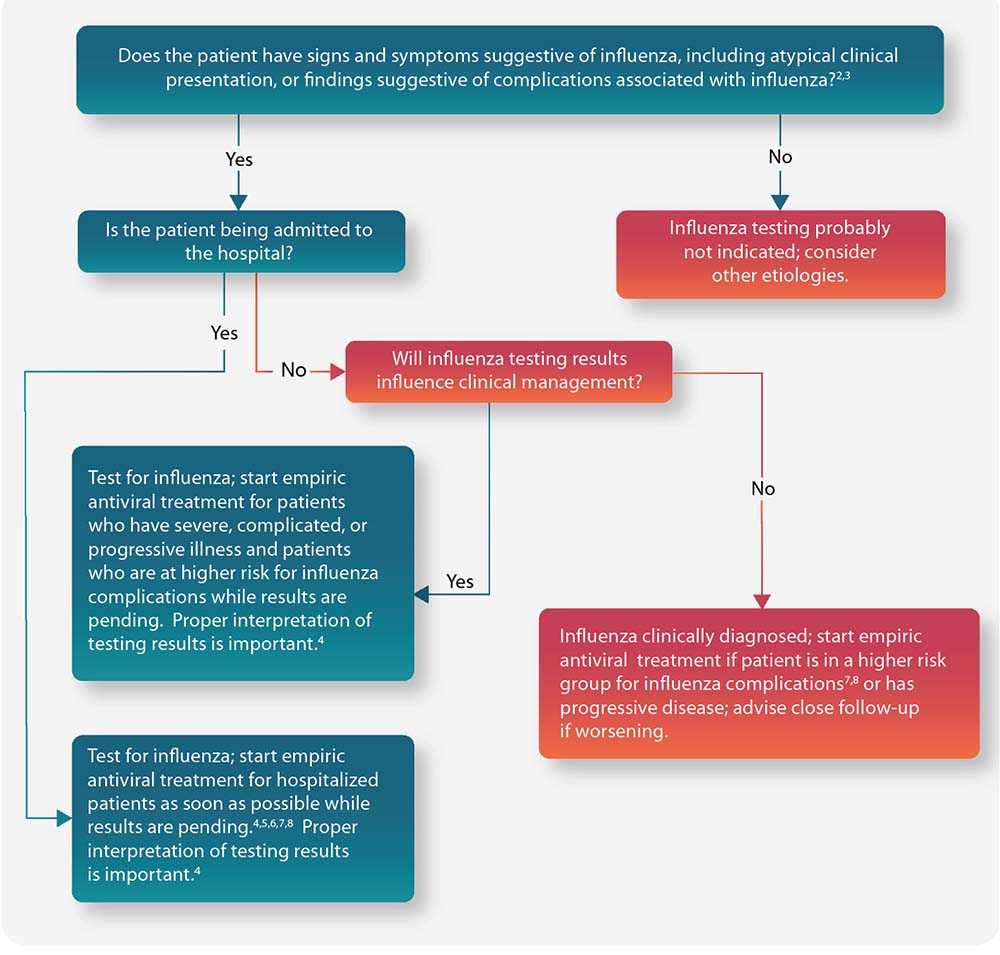
Complete footnotes for this algorithm are available.
People at Higher Risk for Influenza Complications Recommended for Antiviral Treatment- Adults 65 years and older
- Children younger than 2 years old 1
- Asthma
- Neurologic and neurodevelopment conditions
- Blood disorders (such as sickle cell disease)
- Chronic lung disease (such as chronic obstructive pulmonary disease [COPD] and cystic fibrosis)
- Endocrine disorders (such as diabetes mellitus)
- Heart disease (such as congenital heart disease, congestive heart failure and coronary artery disease)
- Kidney diseases
- Liver disorders
- Metabolic disorders (such as inherited metabolic disorders and mitochondrial disorders)
- People who are obese with a body mass index [BMI] of 40 or higher
- People younger than 19 years old on long-term aspirin- or salicylate-containing medications
- People with a weakened immune system due to disease (such as people with HIV or AIDS, or some cancers such as leukemia) or medications (such as those receiving chemotherapy or radiation treatment for cancer, or persons with chronic conditions requiring chronic corticosteroids or other drugs that suppress the immune system)
- People who have had a stroke
- Pregnant people and people up to 2 weeks after the end of pregnancy
- People who live in nursing homes and other long-term care facilities
- People from certain racial and ethnic minority groups are at increased risk for hospitalization with influenza, including non-Hispanic Black persons, Hispanic or Latino persons, and American Indian or Alaska Native persons. Early antiviral treatment of influenza should be considered for outpatients in these racial and ethnic minority groups
1 Although all children younger than 5 years old are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 years old, with the highest hospitalization and death rates among infants younger than 6 months old. Because many children with mild febrile respiratory illness might have other viral infections (e.g., SARS-CoV-2, respiratory syncytial virus, rhinovirus, parainfluenza virus, or human metapneumovirus), knowledge of other respiratory viruses as well as influenza virus strains circulating in the community is important for treatment decisions.
Treatment Considerations for Patients Hospitalized with Suspected or Confirmed InfluenzaThe following recommendations do not necessarily represent FDA-approved uses of antiviral products but are based on published observational studies and expert opinion and are subject to change as the developmental status of investigational products and the epidemiologic and virologic features of influenza change over time.
- For hospitalized patients with suspected or confirmed influenza, initiation of antiviral treatment with oral or enterically administered oseltamivir is recommended as soon as possible. Antiviral treatment might be effective in reducing morbidity and mortality in hospitalized influenza patients, especially adults, even if treatment is started more than 48 hours after onset of illness.
- Inhaled zanamivir, oral baloxavir, and intravenous peramivir are not recommended routinely for hospitalized patients with suspected or confirmed influenza because of insufficient data on use of these antivirals showing clinical benefit in hospitalized influenza patients. There are also insufficient data for treatment of hospitalized influenza patients with intravenous peramivir.
- The optimal duration and dosing of antiviral treatment are uncertain for severe or complicated influenza. Treatment regimens might need to be altered to fit the clinical circumstances.
- Decisions about extended (longer) duration of treatment should be guided by clinical judgment in patients whose illness is prolonged.
- Virologic testing of lower respiratory tract specimens by real-time reverse transcription-polymerase chain reaction (RT-PCR) can help guide decisions about extended treatment in hospitalized influenza patients with severe and prolonged illness. Critically ill patients with respiratory failure can have prolonged influenza viral replication in the lower respiratory tract and might benefit from longer duration of treatment.
- Longer treatment regimens might be necessary in immunocompromised patients who may have prolonged influenza viral replication. Such patients are at risk of emergence of influenza viruses with reduced susceptibility or antiviral resistance during or after antiviral treatment.
- Limited data suggest that oseltamivir administered orally or by oro/naso gastric tube is well absorbed in critically ill influenza patients, including those receiving continuous renal replacement therapy, and/or on extracorporeal membrane oxygenation (Ariano, 2010 ; Eyler, 2012a ; Eyler, 2012b ; Giraud, 2011 ; Kromdijk, 2013 ; Lemaitre, 2012 ; Mulla, 2013 ; Taylor, 2008 ).
- If a hospitalized patient treated with oseltamivir or peramivir manifests progressive lower respiratory symptoms, resistant virus should be considered. However, clinicians should note that failure to improve or clinical deterioration during oseltamivir or peramivir treatment is more likely to be related to the natural history of acute lung injury and inflammatory damage or onset of other complications (e.g., renal failure, septic shock, ventilator-associated pneumonia) than to emergence of oseltamivir or peramivir resistance.
- Careful attention to ventilator and fluid management and to the prevention and treatment of secondary bacterial pneumonia (e.g., S.pneumoniae, S. pyogenes, and S. aureus, including MRSA) also is critical for severely ill patients (Bautista, 2010 ; Finelli, 2008 ; Hageman, 2006 ; Harper, 2009; Mandell, 2007; Mauad, 2010 ; Shieh, 2010 ).
Table 2. Recommended Dosage and Duration of Influenza Antiviral Medications for Treatment or Chemoprophylaxis
Antiviral Agent
Antiviral AgentAntiviral Agent
Use
UseUse
Children
ChildrenChildren
Adults
AdultsAdults
Antiviral AgentTreatment (5 days) 1
UseTreatment (5 days) 1
If younger than 1 yr old 2 : 3 mg/kg/dose twice daily 3,4 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg twice a day >15 to 23 kg, the dose is 45 mg twice a day >23 to 40 kg, the dose is 60 mg twice a day >40 kg, the dose is 75 mg twice a day
ChildrenIf younger than 1 yr old 2 : 3 mg/kg/dose twice daily 3,4 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg twice a day >15 to 23 kg, the dose is 45 mg twice a day >23 to 40 kg, the dose is 60 mg twice a day >40 kg, the dose is 75 mg twice a day
75 mg twice daily
Adults75 mg twice daily
Antiviral AgentChemoprophylaxis (7 days) 5
UseChemoprophylaxis (7 days) 5
If child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless situation is judged critical due to limited data in this age group. If child is 3 months or older and younger than 1 yr old 2 3 mg/ kg/dose once daily 3 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg once a day >15 to 23 kg, the dose is 45 mg once a day >23 to 40 kg, the dose is 60 mg once a day >40 kg, the dose is 75 mg once a day
ChildrenIf child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless situation is judged critical due to limited data in this age group. If child is 3 months or older and younger than 1 yr old 2 3 mg/ kg/dose once daily 3 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg once a day >15 to 23 kg, the dose is 45 mg once a day >23 to 40 kg, the dose is 60 mg once a day >40 kg, the dose is 75 mg once a day
75 mg once daily
Adults75 mg once daily
Inhaled Zanamivir 6
Antiviral AgentInhaled Zanamivir 6
Treatment (5 days)
UseTreatment (5 days)
10 mg (two 5-mg inhalations) twice daily
Children
(FDA approved and recommended for use in children 7 yrs or older)10 mg (two 5-mg inhalations) twice daily
(FDA approved and recommended for use in children 7 yrs or older)10 mg (two 5-mg inhalations) twice daily
Adults10 mg (two 5-mg inhalations) twice daily
Antiviral AgentChemoprophylaxis (7 days) 5
UseChemoprophylaxis (7 days) 5
10 mg (two 5-mg inhalations) once daily
Children
(FDA approved for and recommended for use in children 5 yrs or older)10 mg (two 5-mg inhalations) once daily
(FDA approved for and recommended for use in children 5 yrs or older)10 mg (two 5-mg inhalations) once daily
Adults10 mg (two 5-mg inhalations) once daily
Intravenous Peramivir 7
Antiviral AgentIntravenous Peramivir 7
Treatment (1 day) 1
UseTreatment (1 day) 1
(6 months to 12 yrs of age) One 12 mg/kg dose, up to 600 mg maximum, via intravenous infusion for a minimum of 15 minutes
(FDA approved and recommended for use in children 6 months or older)
Children(6 months to 12 yrs of age) One 12 mg/kg dose, up to 600 mg maximum, via intravenous infusion for a minimum of 15 minutes
(FDA approved and recommended for use in children 6 months or older)
(13 yrs and older) One 600 mg dose, via intravenous infusion for a minimum of 15 minutes
Adults(13 yrs and older) One 600 mg dose, via intravenous infusion for a minimum of 15 minutes
Antiviral AgentChemoprophylaxis 8
UseChemoprophylaxis 8
Children AdultsOral Baloxavir 9
Antiviral AgentOral Baloxavir 9
Treatment (1 day)
UseTreatment (1 day)
FDA approved and recommended for use in otherwise healthy children 5 yrs and older.
ChildrenFDA approved and recommended for use in otherwise healthy children 5 yrs and older.
Weight Weight ≥80 kg: One 80 mg dose 9
AdultsWeight Weight ≥80 kg: One 80 mg dose 9
Antiviral AgentChemoprophylaxis 9
UseChemoprophylaxis 9
FDA approved for post-exposure prophylaxis for persons aged 5 years and older. Dosage is the same as for treatment.
ChildrenFDA approved for post-exposure prophylaxis for persons aged 5 years and older. Dosage is the same as for treatment.
Dosage is the same as to treatment
AdultsDosage is the same as to treatment
Abbreviations: N/A = not approved
- Longer treatment duration may be needed for severely ill patients.
- Oral oseltamivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset with twice-daily dosing in people 14 days and older, and for chemoprophylaxis with once-daily dosing in people 1 year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year of age, is recommended by CDC and the American Academy of Pediatrics (Recommendations for Prevention and Control of Influenza in Children, 2023–2024 ).
- This is the FDA-approved oral oseltamivir treatment dose for infants 14 days and older and less than 1 year old and provides oseltamivir exposure in children similar to that achieved by the approved dose of 75 mg orally twice daily for adults, as shown in two studies of oseltamivir pharmacokinetics in children (Kimberlin, 2013 [CASG 114], EU study WP2284 [3.44 MB, 74 pages] , FDA Clinical Pharmacology Review [1.7 MB, 53 pages] ). The American Academy of Pediatrics has recommended an oseltamivir treatment dose of 3.5 mg/kg orally twice daily for infants 9-11 months old, on the basis of data which indicated that a higher dose of 3.5 mg/kg was needed to achieve the protocol-defined targeted exposure for this cohort as defined in the CASG 114 study (Kimberlin, 2013 ). It is unknown whether this higher dose will improve efficacy or prevent the development of antiviral resistance. However, there is no evidence that the 3.5 mg/kg dose is harmful or causes more adverse events to infants in this age group.
- Current weight-based dosing recommendations are not appropriate for premature infants. Premature infants might have slower clearance of oral oseltamivir because of immature renal function, and doses recommended for full-term infants might lead to very high drug concentrations in this age group. CDC recommends dosing as also recommended by the American Academy of Pediatrics (Recommendations for Prevention and Control of Influenza in Children, 2023–2024 ): limited data from the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group provide the basis for dosing preterm infants using their postmenstrual age (gestational age + chronological age): 1.0 mg/kg/dose, orally, twice daily, for those 40 weeks postmenstrual age.
- See Special Considerations for Institutional Settings section below for details regarding duration of chemoprophylaxis for outbreaks in institutional settings.
- Inhaled zanamivir is approved for treatment of acute uncomplicated influenza within 2 days of illness onset with twice-daily dosing in people aged ≥7 years, and for chemoprophylaxis with once-daily dosing in people aged ≥5 years.
- Intravenous peramivir is approved for treatment of acute uncomplicated influenza within 2 days of illness onset with a single dose in people aged ≥6 months. Daily dosing for a minimum of 5 days was used in clinical trials of hospitalized patients with influenza (de Jong, 2014 , Ison, 2014 ).
- There are no data for use of peramivir for chemoprophylaxis of influenza.
- Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people aged ≥5 years who are otherwise healthy, or in people aged ≥12 years at high risk of developing influenza-related complications. Baloxavir marboxil (Xofluza) [package insert] [445 KB, 16 pages] . Baloxavir marboxil should not be administered with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc); co-administration with polyvalent cation-containing products may decrease plasma concentrations of baloxavir which may reduce efficacy. There are no available published data from clinical trials for baloxavir treatment of influenza in non-hospitalized patients who are pregnant, immunocompromised, or have severe disease.
A randomized clinical trial reported that combination neuraminidase inhibitor (primarily oseltamivir) and baloxavir for treatment of hospitalized influenza patients aged ≥12 years did not result in superior clinical benefit (time to clinical improvement) compared with neuraminidase inhibitor and placebo (Kumar, 2022).
Influenza Antiviral Resistance Considerations- Antiviral resistance and reduced susceptibility to the neuraminidase inhibitors and to baloxavir among circulating influenza viruses is currently very low, but this can change.
- For weekly surveillance data on susceptibility of circulating influenza viruses to antivirals in the U.S. this season, see the FluView Weekly U.S. Influenza Surveillance Report.
- Oseltamivir resistance in influenza A(H3N2) and A(H1N1)pdm09 viruses can develop during treatment, particularly in young children (Roosenhoff, 2019 ; Lina, 2018 ;), and immunocompromised persons (Memoli, 2014) .
- Influenza viruses may become less susceptible or resistant to oseltamivir and peramivir during antiviral treatment with one of these drugs and remain susceptible to zanamivir; this has been reported most often for influenza A(H1N1)pdm09 viruses (Graitcer, 2011; Lackenby, 2011; Memoli, 2010; Nguyen, 2010; Nguyen, 2012).
- Influenza A(H1N1)pdm09 viruses have also emerged that are resistant to all neuraminidase inhibitors, including zanamivir, in highly immunosuppressed patients on prolonged neuraminidase inhibitor treatment (Tamura, 2015 ; L’Huillier, 2015 ).
- Human-to-human transmission of influenza A(H1N1)pdm09 viruses with an H275Y mutation in viral neuraminidase conferring resistance to oseltamivir has been reported among severely immunocompromised patients in hospital units, (Gooskens, 2009 ; Chen, 2011 ;) and in the community (Hibino, 2017 ; Le, 2008;Hurt, 2011 ; Hurt, 2012 ; Takashita, 2013) , but currently appears to be uncommon.
- Following treatment with baloxavir, emergence of viruses with molecular markers associated with reduced susceptibility to baloxavir has been observed in clinical trials in immunocompetent children and adults, with higher detection among baloxavir-treated pediatric patients aged
- Limited human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir has been reported sporadically in Japanese children (Takashita, 2019 ; Takashita 2019 ; Imai, 2019 ), but currently appears to be uncommon.
- Molecular analyses can detect genetic changes in influenza viruses associated with resistance and reduced susceptibility to oseltamivir and peramivir. The CDC Influenza Division is available for consultation regarding antiviral susceptibility testing as needed. Information about neuraminidase inhibitor susceptibility testing and interpretation of results of neuraminidase inhibition assays is available on the WHO website .
Patients with Uncomplicated Influenza
- Meta-analyses of randomized controlled clinical trials (RCTs) have demonstrated efficacy of early initiation of treatment (started within 36 to 48 hours of illness onset) with neuraminidase inhibitors in reducing duration of fever and illness symptoms compared with placebo in otherwise healthy children and adults with uncomplicated influenza ( Jefferson, 2014 ; Dobson, 2015 ; Malosh, 2018 ; Liu, 2021 ).
- One randomized clinical trial in children with uncomplicated influenza demonstrated a modest reduction in duration of symptoms and influenza virus shedding in patients initiating treatment after 48 hours; post hoc analysis suggested that oseltamivir treatment initiated 72 hours after illness onset reduced symptoms by one day compared with placebo (Fry, 2014 ).
Hospitalized Patients
- No completed, sufficiently powered, randomized, placebo-controlled clinical trials have been conducted of monotherapy with neuraminidase inhibitors for treatment of influenza in hospitalized patients; studies supporting the licensure of oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir were conducted in outpatients, primarily among previously healthy persons with uncomplicated illness.
- A secondary analysis of a multi-center unblinded clinical trial of oseltamivir treatment started within 24 hours of enrollment after hospital admission versus standard of care in adults hospitalized for lower respiratory tract infection reported that oseltamivir treatment lowered the risk of clinical failure in patients with laboratory-confirmed influenza; clinical failure was defined as failure to improve with 7 days, transfer to ICU care 24 hours after admission, or rehospitalization or death within 30 days (Wiemken, 2021 ).
- In pregnant people, antiviral treatment in any trimester with influenza A(H1N1)pdm09 virus infection has been shown to be most beneficial in preventing respiratory failure and death when started within 2 days of illness (Siston, 2010 ).
- Observational studies in hospitalized patients with influenza have reported greater clinical benefit when oseltamivir or other neuraminidase inhibitor treatment are started at or promptly after hospital admission compared with later treatment initiation or no antiviral treatment (Katzen, 2018 , Venkatesan, 2019 ).
- Observational studies in hospitalized adult patients with influenza have reported that starting oseltamivir treatment within 48 hours of hospital admission can reduce ICU admission, 30-day readmissions and mortality compared with no treatment or later initiation of treatment (Sharma 2021 , Groeneveld 2020 ).
- Treatment: Recommended duration for antiviral treatment of uncomplicated influenza in outpatients is 5 days for oral oseltamivir or inhaled zanamivir. For treatment of uncomplicated influenza with intravenous peramivir or oral baloxavir, a single dose is recommended. Longer daily dosing (oral oseltamivir or intravenous peramivir) can be considered for hospitalized patients with influenza who remain severely ill after 5 days of treatment. Treatment should be started as soon as possible after symptom onset for the greatest clinical benefit.
- Chemoprophylaxis: Recommended duration is 7 days (after last known exposure). For control of outbreaks in institutional settings (e.g., long-term care facilities for older adults and children) and hospitals, CDC recommends antiviral chemoprophylaxis of exposed residents with oral oseltamivir or inhaled zanamivir for a minimum of 2 weeks and continuing up to 1 week after the last known case was identified. Antiviral chemoprophylaxis is recommended for all residents, including those who have received influenza vaccination. For control of some institutional influenza outbreaks, post-exposure antiviral treatment has been used (e.g., oseltamivir twice daily for 5 days) instead of post-exposure antiviral chemoprophylaxis (Uyeki, 2019 ). Baloxavir is approved for post-exposure prophylaxis (single dose) of influenza in persons aged 5 years and older within 48 hours of contact with an individual with influenza.
Dose adjustment of oseltamivir is recommended for patients with creatinine clearance between 10 and 60 mL/min and patients with end-stage renal disease (ESRD) undergoing hemodialysis or continuous peritoneal dialysis receiving oseltamivir for the treatment or chemoprophylaxis of influenza. Oseltamivir is not recommended for patients with ESRD not undergoing dialysis. The recommended doses are detailed in Table 3; duration of treatment and chemoprophylaxis is the same as recommended for patients with normal renal function. The dose of intravenous peramivir should be reduced for patients with baseline creatinine clearance below 50 mL/min (see Table 3).
No dose adjustment is recommended for inhaled zanamivir for a 5-day course of treatment for patients with renal impairment. Pharmacokinetic analysis did not identify a clinically meaningful effect of renal function on the pharmacokinetics of baloxavir in patients with creatinine clearance 50 mL/min and above. The effects of severe renal impairment on the pharmacokinetics of baloxavir marboxil or its active metabolite, baloxavir, have not been evaluated.